Description
DESCRIPTION
White to grayish tablets with a specific smell of the ingredients.
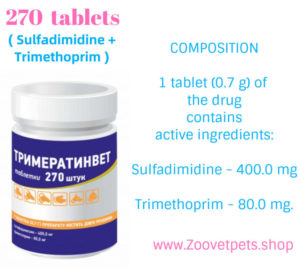
COMPOSITION
1 tablet (0.7 g) of the drug
contains
active ingredients:
Sulfadimidine – 400.0 mg
Trimethoprim – 80.0 mg.
PHARMACOLOGICAL PROPERTIES
ATC vet classification code QJ01 – antimicrobial veterinary drugs for systemic use. QJ01EW03 – combination of sulfonamides and trimethoprim. Sulfadimidine (sulfadimesin) and trimethoprim.
Trimeratinvet tablets is a combination drug consisting of sulfadimidine and trimethoprim. The effectiveness of the drug is due to the action of the above components, which in combination exhibit a synergistic effect, resulting in significantly higher activity of the drug than the activity of the individual components.
Sulfadimidine and trimethoprim act bacteriostatically on most gram-positive and gram-negative microorganisms, including: Staphylococcus spp., Streptococcus spp., Salmonella spp., E. coli, Pasteurella spp., Serpulina hyodysenteriae, Corynebacterium spp., Erysipelothrix rhusiopathiae.
The mechanism of action is the sequential inhibition of enzymes involved in folic acid synthesis, thereby inhibiting thymidine synthesis of the bacterial cell.
Sulfadimidine blocks the conversion of para-aminobenzoic acid to dihydrofolic acid, while trimethoprim blocks the conversion of dihydrofolic acid to tetrahydrofolic acid by inhibiting dihydrofolate reductase.
Sulfadimidine and trimethoprim are well distributed throughout the body and penetrate through the placenta. In inflammation of the cerebral membranes, the concentration can reach 50% of the serum concentration.
Sulfadimidine and trimethoprim are excreted through the kidneys by glomerular filtration and tubular secretion, metabolized in the liver.
Sulfadimidine is mainly acetylated and conjugated to glucuronic acid, trimethoprim is metabolized to oxide and hydroxylated metabolites.
INDICATIONS FOR USE
Treatment of cattle, sheep, goats, pigs, dogs, cats and poultry (broiler chickens and young laying hens) for diseases of digestive tract, respiratory and urogenital systems and complications of viral diseases, postoperative and posttraumatic infections caused by microorganisms sensitive to trimethoprim and sulfadimidine.
DIRECTIONS FOR USE AND DOSAGES
Orally, dissolved in water, milk, milk replacer or mixed with feed in doses:
– cattle – 1 tablet per 44-77lb (20-35 kg) of animal body weight;
– sheep, goats – 1 tablet per 33-44lb (15-20kg) of body weight;
– pigs, calves – 1 tablet per 33-44lb (15-20kg) of the animal’s body weight;
– dogs, cats – 1 tablet per 33-44lb (15-20 kg) of body weight.
Applied at intervals of 24 hours, the treatment course of 3-5 days.
CONTRAINDICATIONS .
Hypersensitivity to sulfadimidine and trimethoprim.
Do not use in animals with impaired liver and kidney function as well as in case of blood dyscrasia.
Do not use in laying hens whose eggs are used for human consumption.
CAUTION
Slaughter of animals and poultry for meat is allowed within 7 days (cattle, sheep, goats, pigs) and 2 days (poultry) after the last drug administration. Consumption of milk for human consumption shall be allowed 3 days after the last drug administration. Meat and milk obtained before the specified period shall be utilized or fed to unproductive animals, depending on the opinion of a veterinarian.
STORAGE
FROM 40-77F (5°C to 25°C), dry, dark place, out of reach of children.
SHELF LIFE – 3 years.








Reviews
There are no reviews yet.